


Sighture's intelligent catheter system revolutionizes urological monitoring by integrating three critical functions into a single sterile device. It provides real-time core body temperature readings via bladder-placed thermal sensors, continuous intravesical pressure monitoring, and automated urine output measurement through optical scanning technology. This integrated approach eliminates the need for multiple separate monitoring devices, significantly improving workflow efficiency in ICU and postoperative settings. The system's closed-circuit design prevents contamination while ensuring measurement accuracy, with all data displayed simultaneously on connected monitoring equipment for comprehensive patient assessment.
Critical Care Monitoring - Continuous bladder pressure and core temperature tracking in ICU
Postoperative Management - Early detection of urinary retention after surgery
Precise Fluid Management - Automated urine output measurement with 97% accuracy
Burn Patient Care - Reliable core temperature monitoring alternative
This integrated system replaces multiple separate devices, providing comprehensive urological monitoring in critical care settings.
Sighture implements a multi-stage quality control protocol meeting ISO 13485 standards. Each catheter undergoes material certification for medical-grade sensors and biocompatible silicone. Assembly occurs in controlled cleanroom environments with electrostatic protection. Finished products complete 12 performance validations including temperature accuracy (±0.2°C), pressure calibration (0-300cmH₂O), and optical urine monitoring precision. Sterility is ensured through validated EO sterilization cycles. Final inspection includes packaging integrity verification and electrical safety testing. This comprehensive process guarantees reliable performance and patient safety in critical care applications.

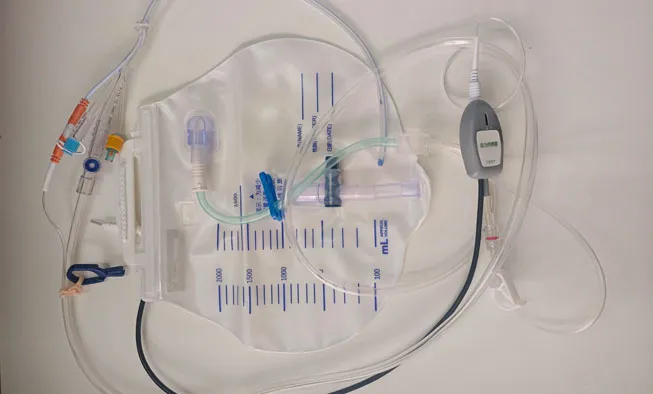
1. Preparation - Assess patient and prepare sterile equipment using aseptic technique
2. Insertion - Lubricate catheter tip, gently advance through urethra until urine flow observed
3. Positioning - Inflate retention balloon with sterile water after proper placement confirmation
4. Connection - Attach catheter to monitoring unit and drainage system using closed-circuit method
5. Monitoring - Connect sensors to display unit for continuous temperature, pressure and urine output tracking
6. Documentation - Record initial measurements and establish baseline monitoring parameters
The complete sterile procedure requires approximately 10-15 minutes, with continuous monitoring capabilities maintained throughout catheterization period.

The system maintains precision of ±0.2°C for temperature and ≤1.5% error for pressure monitoring, as validated through clinical testing and based on the specifications you provided.

Yes, it features universal connectors that interface with most ICU monitors and electronic medical record systems, facilitating seamless integration into existing clinical workflows.

The catheter is designed for single-use. The specific duration of use should be determined by healthcare professionals based on the patient's clinical condition and adherence to hospital protocols for indwelling catheters.

Please refer to the product packaging and official documentation for specific storage conditions and shelf life to ensure product integrity and performance.

Upgrade your medical practice with Sighture's cutting-edge Diagnostic & Therapeutic Equipment, including advanced imaging systems, precision laser therapies, and AI-powered diagnostic tools. Our FDA/CE-certified medical devices deliver unmatched accuracy for ophthalmology, dermatology, and rehabilitation applications. Contact us today for tailored solutions that combine innovation with clinical reliability.